Phase transition and metastability |
|
Phase transitions are involved in many key physical processes and we seek a microscopic understanding of their role. Our studies address a variety of systems, that includes the metastability of supercooled water, the condensation in porous materials, the dissolution and cristallisation of minerals, the evaporation of droplets and the solidification processes in frozen materials. |
Members: Brall, Niels |
||
Highlights |
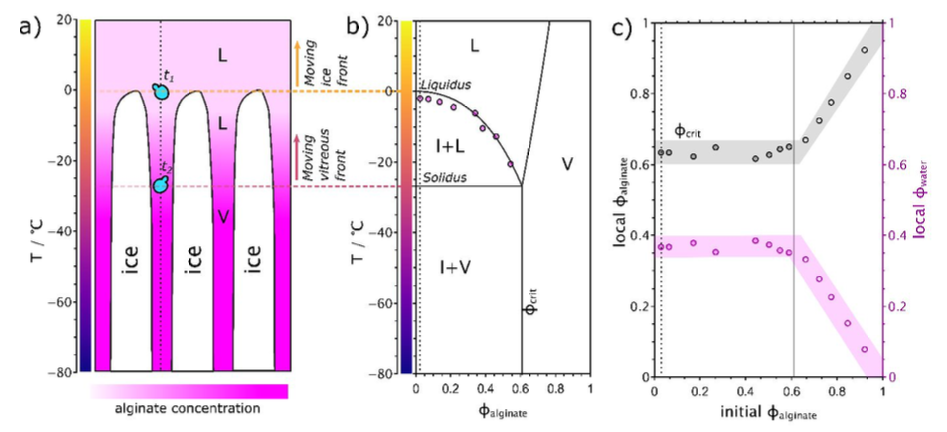
Unveiling cells’ local environment during cryopreservationWe couple in situ microscopic directional freezing to visualize cells and their surroundings during freezing with the freezing-medium phase diagram. Our correlative strategy may be applied to cells relevant to clinical research and practice and may help in the design of new cryoprotective media based on local physicochemical cues. Unveiling cells’ local environment during cryo-preservation... Qin et al., J Phys Chem Lett (2020) |
|
Phase segregation of alginate/water solution (L) during directional freezing enables controlled phase separation into ice (I) and a vitreous phase (V).
|
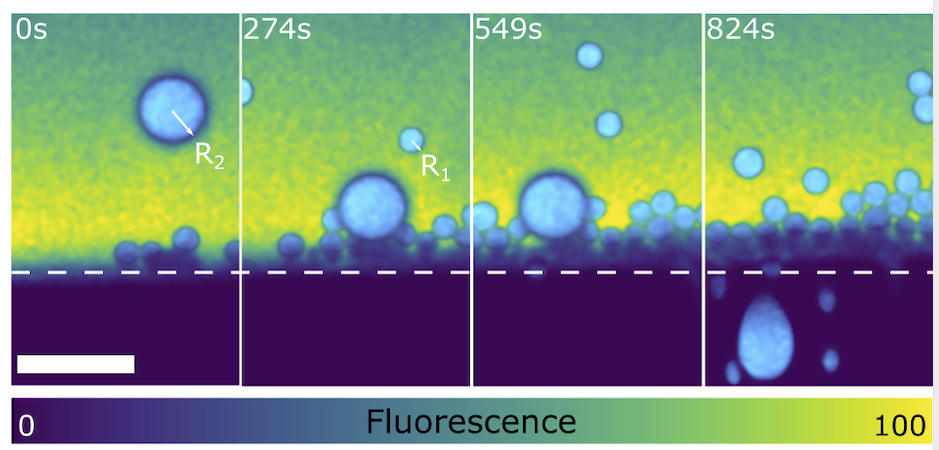
Objects interacting with a solidification frontWe show the interaction of multiple oil droplets with an ice-water front in the absence and presence of solute effects using in situ cryo-confocal microscopy. We report on how the object size, number of objects, and bulk solute concentration influence the the object-front interaction and the front morphology, as well as the subsequent object spatial distribution. Multiple objects interacting with a solidification front, Tyagi et al., Scientific Reports (2020). |
|
Rejection or encapsulation of oil droplets of different sizes by the front |
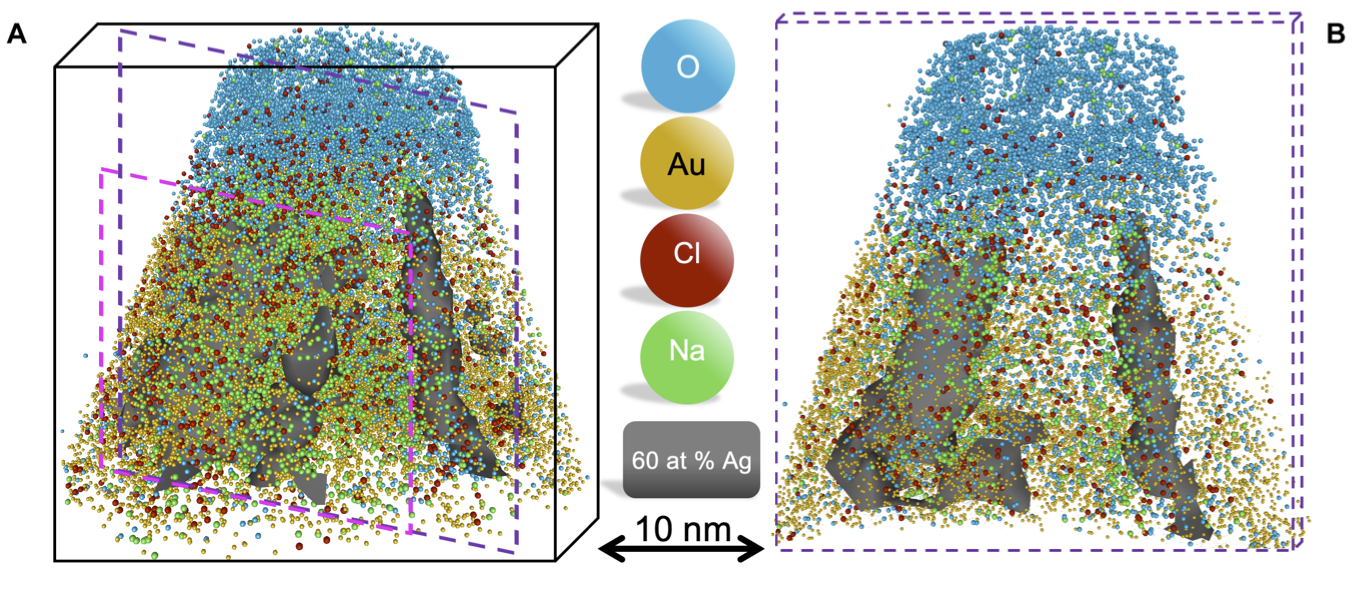
Atome probe tomography of frozen liquidsWe used atom probe tomography to analyze frozen liquids in three dimensions with subnanometer resolution. Our study demonstrates the viability of using frozen water as a carrier for near-atomic–scale analysis of objects in solution by atom probe tomography. Enabling near-atomic–scale analysis of frozen water, El-Zoka et al., Science Advances (2020) |
|
|
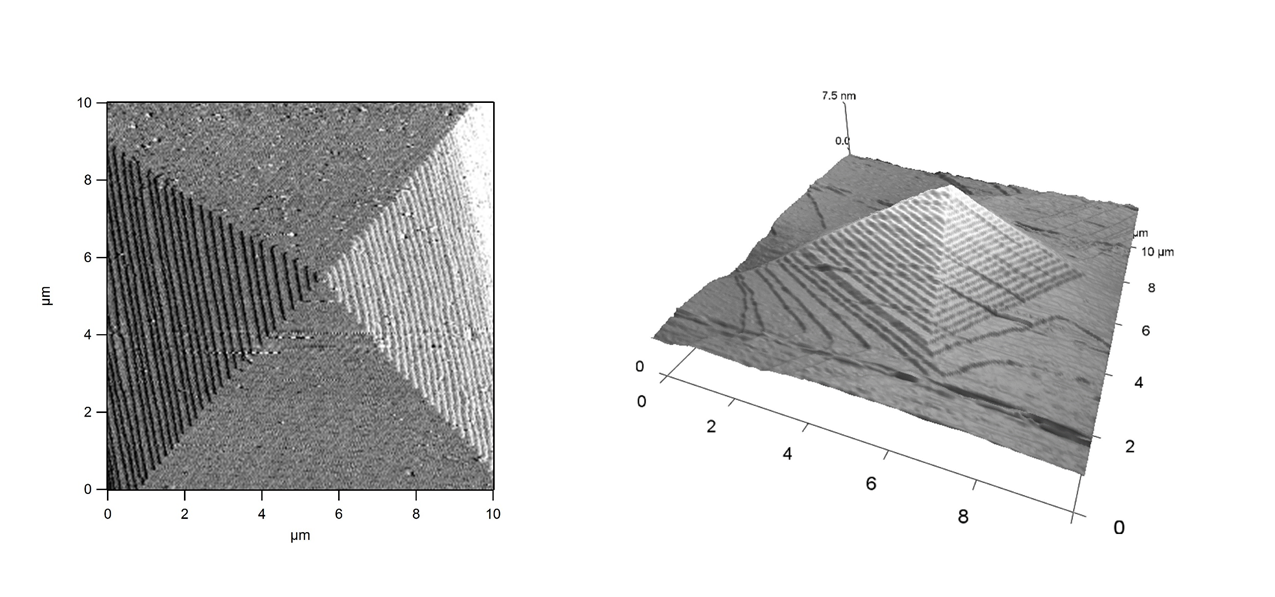
Calcite growth under stressWe have shown, with Atomic Force Microscopy measurements, that the application of a stress during the growth of a calcite crystal induces a slowdown of the growth kinetics, and even a change of the growing crystalline phase. Tuning biotic and abiotic calcite growth by stress, Zareeipolgardani et al., Crystal Growth Des. (2019). |
|
|
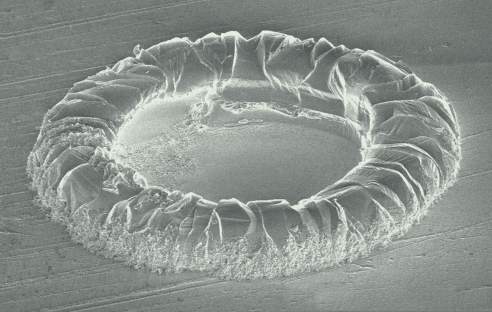
Salt tunnels formed by the evaporation of a dropWe have observed thin salt shells that form at the periphery of evaporating pure water drops on salt. Shell shapes range from rings of inclined walls to hollow toroidal rims. We have interpreted this phenomenon as a consequence of a molecular coffee-stain effect by which the dissolved salt is advected toward the pinned contact line where an increased evaporation drives the crystallization of the shell. Hollow rims from water frop evaporation on salt substrates, Mailleur et al., Phys. Rev. Lett. (2018) |
Scanning electron micrograph of a hollow rim formed in water drop evaporation on a single crystalline NaCl substrate. |
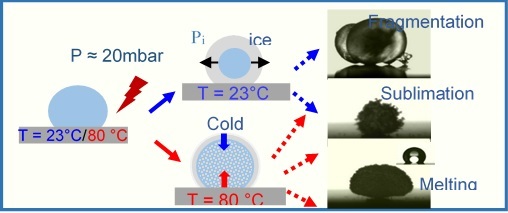
Freezing water droplets on heated highly hydrophobic surfaceWe experimentally study the freezing process of water drops and its temporal stability on highly hydrophobic surfaces. Main results: (i) three different mechanisms influenced by the substrate’s temperature, lead to the disappearance of the frozen droplets. A “Cassie ice state” can be reached. (ii) instabilities and convective cells are observed on heated substrates. Stability of frozen water droplets on highly hydrophobic..., Ramos et al., Appl. Surf. Sc. (2018). |
Summary of main results of this study. Placed on heated substrate the water droplets freeze under low pressure. |
The more, the swifterCommuting during rush hours teaches us that the denser the crowd, the slower the motion. In contrast, increasing pressure in water makes it denser, but less viscous! At 20°C, the effect is modest. By supercooling water to -29°C, we find that viscosity drops by nearly one half for a 2000 atm pressure increase. We propose an explanation based on a two-state model. Pressure dependence of viscosity in supercooled water and a unified approach for thermodynamic and dynamic anomalies of water, L.P. Singh et al., PNAS (2017). |
Close-packed fishes flow fast, as supercooled water under pressure. |
Drop evaporation on superhydrophobic surfacesWe experimentally study the evaporation of drops on heated superhydrophobic surfaces decorated with micrometer-sized mushroom-like pillars.The drop evaporation appears to be controled by the contact line dynamics. Main results (i) in the pinned regime, the substrate heating promotes the contact line depinning; (ii) in the moving regime, the droplet motion is described by periodic stick−slip events and contact-angle oscillations (iii) remarkable stability of the “fakir” state to the temperature. Water drop evaporation on mushroom-like super-hydrophobic surfaces..., Marcelo do Nascimento et al., Langmuir (2016) |
SEM image of a typical investigated sample and snapshots of the top views of an evaporating sessile drop, |
More highlights |