The physics of lipid membranes
Investigator: Jean-Paul Rieu
Role of phospholipid bilayers in biolubrication
The mechanism of boundary lubrication of articular joint, which leads to exceptionally low friction coefficients, is still poorly understood especially if one has in mind that cartilage is very rough and the joint is filled with a complex synovial fluid made of several carbohydrates and proteoglycans, proteins and lipid membranes (both stacks of membranes on cartilage and multilamellar vesicles in the fluid). The role of Supported Phospholipid Bilayers (SPBs) in controlling and reducing frictional forces between biological surfaces has been investigated since several years using a home-made tribometer where lipid bilayer degradation is controlled in-situ during friction testing using fluorescence microscopy (collaboration with LaMCoS, INSA Lyon). In parallel, mechanical resistance to indentation is measured by AFM (Fig. A) and the diffusion coefficient of supported bilayers by FRAP (Fluorescence Recovery After Photobleaching).
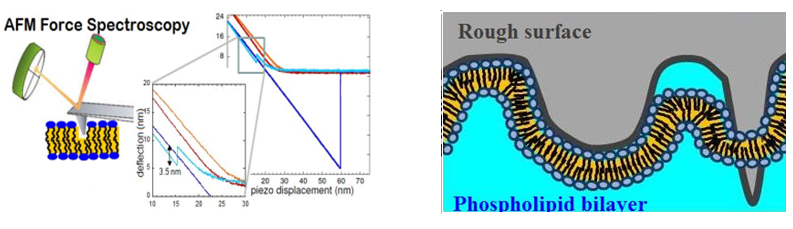
We have shown that it is possible to reduce the friction coefficient of bare surfaces by nearly 2 orders of magnitude, to a very reproducible value µ=0.002 when surfaces are covered by a DPPC SPB (gel phase). With only one bilayer in the contact region (gel or fluid phase), or with two fluid DOPC bilayers in water, the friction coefficient was much higher than with two DPPC bilayers (Trunfio-Sfarghiu et al., 2008 Langmuir). Our study therefore showed that good mechanical stability of the SPB is essential and suggested that the low friction coefficient is ensured by the hydration layers between adjacent lipid bilayers. We also showed than buffer or ions may increase bilayer cohesion and hence decrease wear and friction (Dekkiche et al., 2010 Coll. Surf. B). The effect of roughness on the bilayer structure, diffusion coefficient and tribological parameters is subject to deep experimental and theoretical investigations currently.
Interactions between bilayers and cavitating bubbles in the context of Sonoporation
In the context of intracellular drug delivery for biomedical applications, the transport of bioactive coumpounds (such as fluorescent markers, siRNA, proteins) with low cytotoxicity and damaging effects remains challenging. Without the use of viral vectors, sonoporation, i.e. the permeabilization and poration of the cell membrane by ultrasound technique, enables the delivery of various drugs with minimal immunological responses, with the advantage of a noninvasive spatially targeted in-vivo delivery thanks to the possibility of focusing acoustic energy. However, the mechanisms underlying sonoporation mediated transmembrane and transcellular transport are not well understood. We have used fluorescent SPBs lipid bilayers as a model for biological membranes and have investigated the interactions between the bilayer and microbubbles induced by ultrasound. Among the various types of damages caused by bubbles on the surface, our experiments exhibited a singular dynamic interaction process where bubbles are jumping on the bilayer, forming a necklace pattern of alteration on the membrane (Fig. A, Der Loughian et al., 2015). This phenomenon was explored with different time and space resolutions and, based on our observations, we propose a model for a microbubble subjected to the combined action of Van der Waals, acoustic and hydrodynamic forces. Describing the repeated jumps of the bubble, this model explains the lipid exchanges between bubble and bilayer.