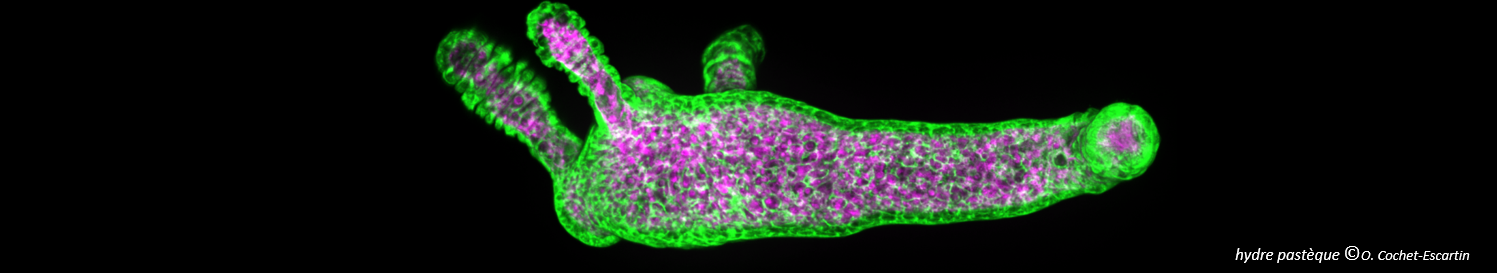
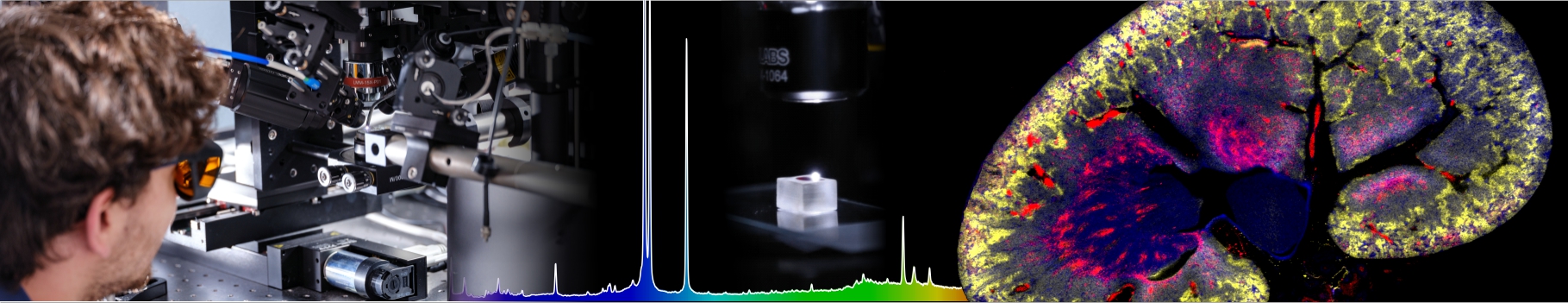
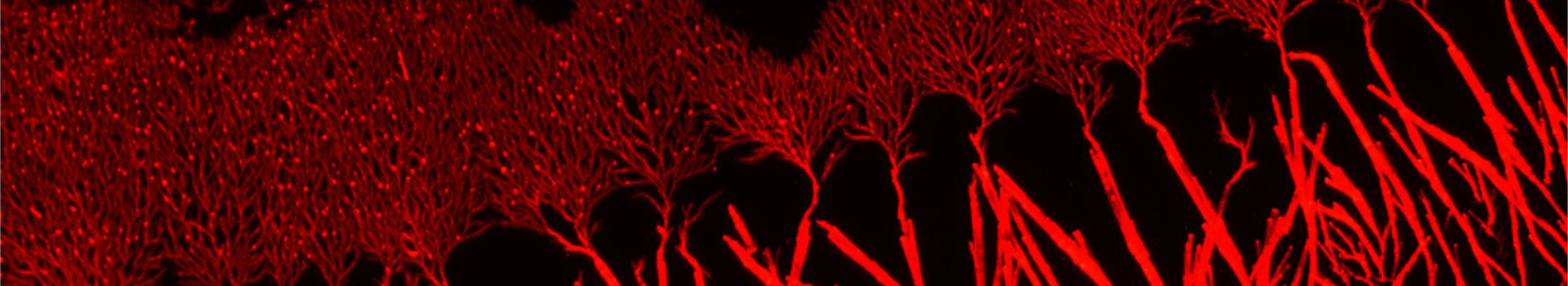
Fibrils or not fibrils...
 Steve Daly, Alex Kulesza and their colleagues from P. Dugourd’s group (team “Spectrobio”) have published a paper entitled "Conformational changes in Amyloid-Beta (12-28) alloforms studied using action-FRET, IMS and molecular dynamics simulations." in the journal Chemical Sciences.
Steve Daly, Alex Kulesza and their colleagues from P. Dugourd’s group (team “Spectrobio”) have published a paper entitled "Conformational changes in Amyloid-Beta (12-28) alloforms studied using action-FRET, IMS and molecular dynamics simulations." in the journal Chemical Sciences.
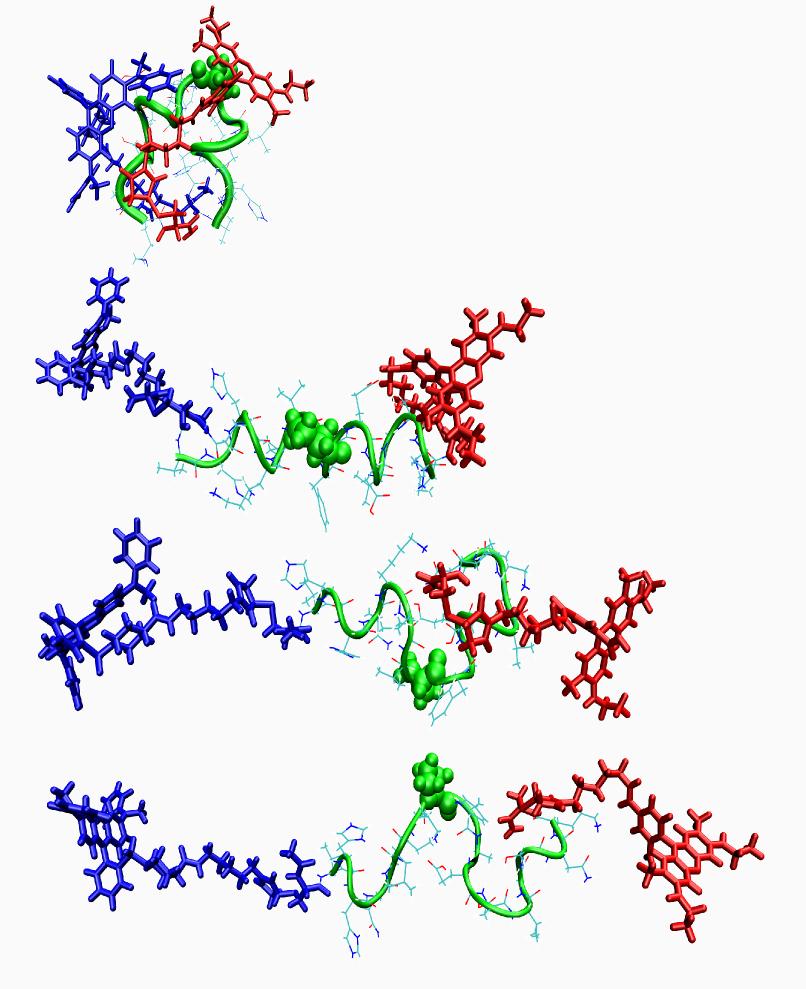 In Alzheimer’s disease, small oligomers of protein β-Amyloïd play the rôle of infectious agents. However, it seems that mutations in the small hydrophobic core formed by amino-acids 17-21 considerably alter the protein propensity to form large fibrils. In order to understand the origins of this behavior, conformation families have been compared for both wild and mutated proteins: under structural stress such as protonation, both forms progressively lose their native structure. However, during this unfolding process, only the wild-type steps through stable intermediates displaying the β-sheet secondary structures involved in pathological fibrils. In order to reach those conclusions, the experimental techniques developed in Dugourd’s group have been combined with a molecular dynamics approach.
In Alzheimer’s disease, small oligomers of protein β-Amyloïd play the rôle of infectious agents. However, it seems that mutations in the small hydrophobic core formed by amino-acids 17-21 considerably alter the protein propensity to form large fibrils. In order to understand the origins of this behavior, conformation families have been compared for both wild and mutated proteins: under structural stress such as protonation, both forms progressively lose their native structure. However, during this unfolding process, only the wild-type steps through stable intermediates displaying the β-sheet secondary structures involved in pathological fibrils. In order to reach those conclusions, the experimental techniques developed in Dugourd’s group have been combined with a molecular dynamics approach.