Cell-Cell interactions and collective motility
Investigators: Jean-Paul Rieu, Charlotte Rivière, Christophe Anjard
The motility of single dicty
Understanding the fundamental mechanisms of collective migration is crucial for investigations of various biological processes such as embryonic morphogenesis, wound repair and cancer invasion. Cells can migrate in loose or tight groups. The emergence of directional migration for loose groups is guided by extrinsic chemotactic and mechanical cues. Compared to the established roles of chemical signal guidance (diffusible factors), the roles of mechanical signals are less known and only now slowly emerging as an important aspect of investigations both experimentally and theoretically
As an experimental system, we use Dictyostelium discoideum (Dd, or simply dicty) one of the best-studied models of cell motility with many of the evolutionary conserved molecular mechanism commonly found in fast moving cells such as immune cells. This model is referenced by the National institute of Health, its genome is entirely sequenced and a large collection of mutants if available for the community at the Stock Center of the dictyBase. We work mostly in nutrient rich conditions (vegetative state), as cells do not express cell-cell adhesion molecules and their basic motility is stable (not evolving with time as in developmental stages), classically described as a persistent random walk in 2D.
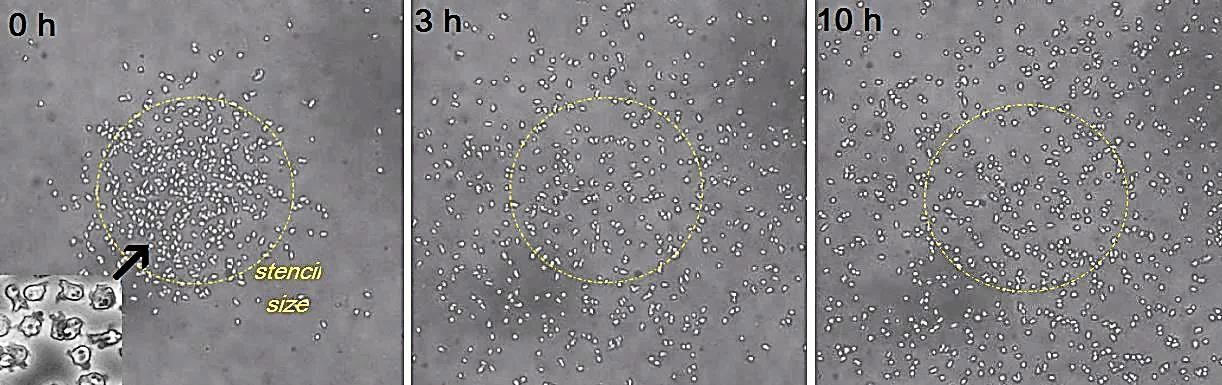
Using 2D motility assays, we have shown that the migration of vegetative Dd cells is regulated by secreted quorum sensing factors (QSF) [1]. From newly developed 2D spreading assay using PDMS stencils, we discovered that the spreading of microcolonies is faster when cell density increases (Fig. 1). At long times, the accumulation of secreted QSF will act to reduce the overall cell motions, thus preventing them to drift too far apart. But at short times, cell-cell collisions seem to induce Contact Enhancement of Locomotion (CEL). These observations look closely related to the concept of ‘Contact Inhibition of Locomotion’ (CIL) described long time ago [2] and recently thoroughly investigated in various cell lines [3]. A deeper understanding of the spreading of Dd, including using simulations and theoretical modelling could pave the way towards a better global understanding of contact interactions in between individual cells.
[1] L. Golé, C. Rivière, Y. Hayakawa, J.-P. Rieu. A quorum-sensing factor in vegetative Dictyostelium cells revealed by quantitative migration analysis. PLoS ONE 6 (2011) e26901.
[2] Abercrombie, M. and Heaysman, J.E.M. (1954) Observations on the social behaviour of cells in tissue culture: II. ‘Monolayering’ of fibroblasts. Exp. Cell Res. 6, 293–306
[3] Mayor R. and Carmona-Fontaine C. Keeping in touch with contact inhibition of locomotion. Trends in Cell Biology 20 (2010) 319–328
Slug motility
Thanks to the traction force microscopy technique based on the measurement of soft gel substrate deformations, we measured few years ago the 3D (x; y; z) force map of Dd slugs The force maps look like the one of a capillary droplet on a surface: forces are directed upward all around the periphery due to contractile peripheral shell tension forces and downward in the inner part due to the resulting internal pressure; the ratio of vertical over tangential forces seems proportional to the tangent of the contact angle. The shell line tension originates from the extracellular matrix made of cellulose and fibrils (slime sheaths) surrounding the slug with a much larger magnitude of ~50 mN/m (Fig. 3B). A continuum scheme with an internal tissue pressure acting against a shell with a lower resistance in the slug tip and a proper grip to the substrate seems to explain the forward propulsion of the slug. The situation is someway analogous to the plant cell growth. However, in plants, the inner pressure is well identified and has an osmotic origin (turgor pressure). Much more work is needed to understand how inner Dd cells build up this internal slug pressure from its unit cell elements. For instance, we have shown that the absence of anterior like cells is dramatic for force transmission to the substrate (Rieu et al., 2009 Cell Mot. Cyto, Fig 2).