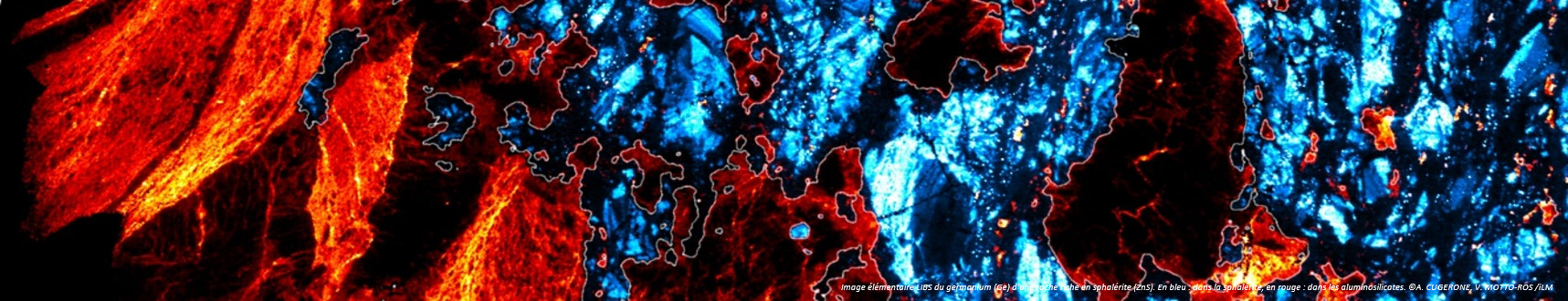
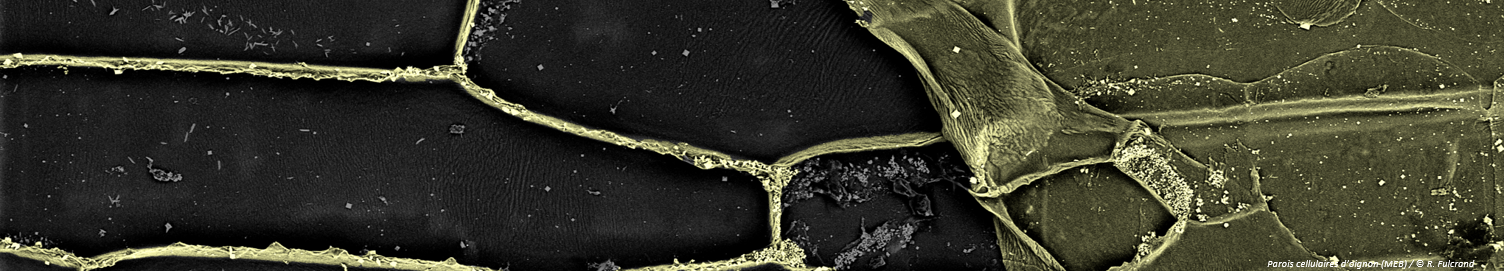
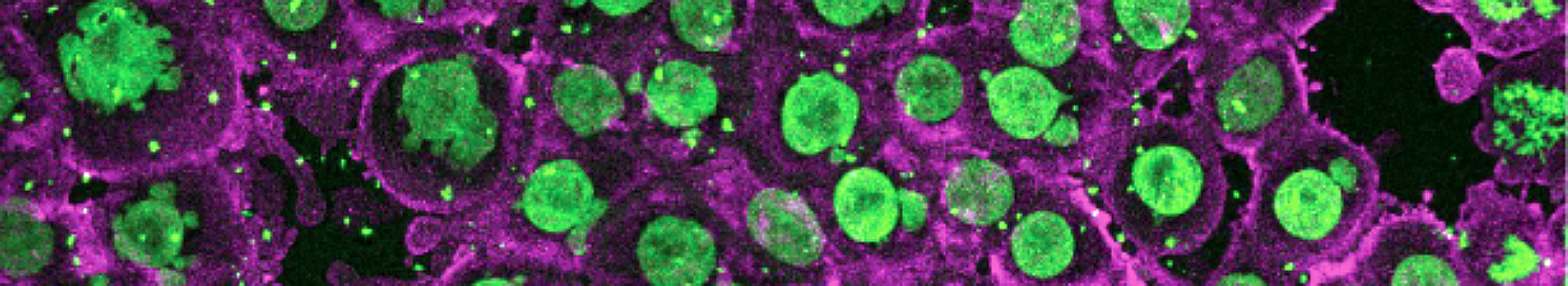
Surface tension: nanobubbles and nanodroplets break the symmetry
Nicolas Bruot and Frédéric Caupin (team Liquides et Interfaces), published an article entitled «Curvature-dependence of the liquid-vapor surface tension beyond the Tolman approximation» in the journal Physical Review Letters.
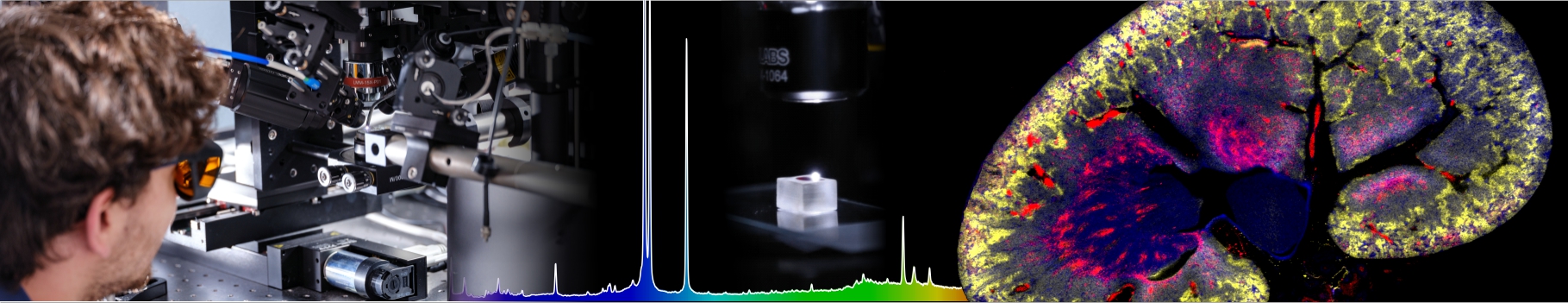
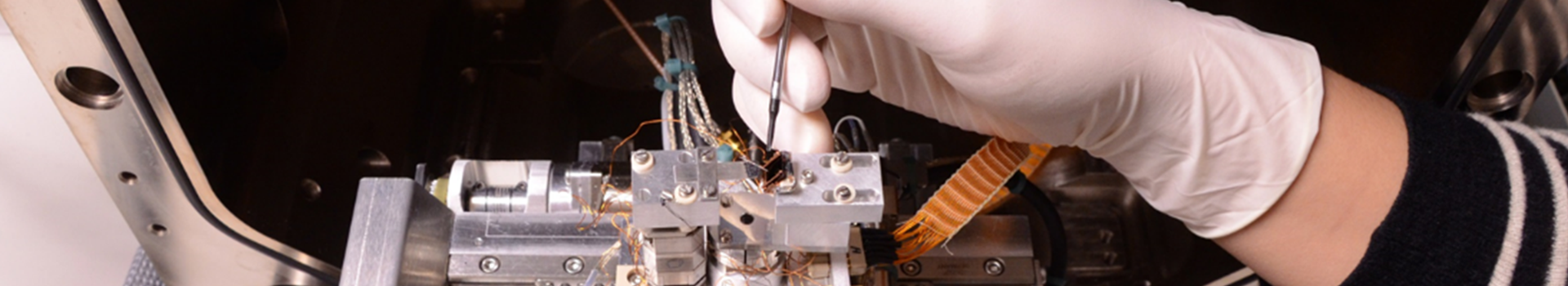
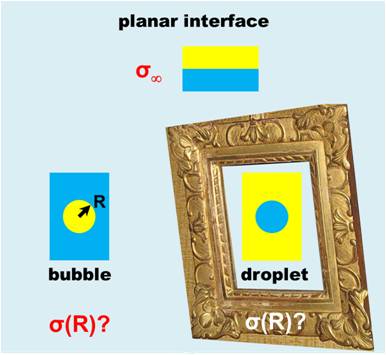 What is the surface tension at the nanoscale? A 66 years old hypothesis made by Tolman proposes to correct the usual, macroscopic surface tension by an amount inversely proportional to the radius of curvature of the liquid-vapor interface. But not without following certain symmetry rule: the correction would be of the same magnitude, but with opposite sign for bubbles and droplets with the same radius. This effect has major implications for nucleation (cavitation of vapor bubbles in a superheated liquid, or condensation of liquid droplets in a supersaturated steam). Indeed, a small change in the surface tension is sufficient to change the nucleation rate by several orders of magnitude. ILM researchers have actually performed cavitation experiments in ethanol and heptane. Combining their measurements with cavitation data in water and condensation data in vapors of ethanol, heptane and water, they were able to show that the symmetric correction proposed by Tolman was not sufficient at the scales relevant to nucleation. This study suggests replacing the Tolman approximation, commonly used in nucleation, with a more complex correction (involving also the square of the radius of curvature) as suggested by recent simulations.
What is the surface tension at the nanoscale? A 66 years old hypothesis made by Tolman proposes to correct the usual, macroscopic surface tension by an amount inversely proportional to the radius of curvature of the liquid-vapor interface. But not without following certain symmetry rule: the correction would be of the same magnitude, but with opposite sign for bubbles and droplets with the same radius. This effect has major implications for nucleation (cavitation of vapor bubbles in a superheated liquid, or condensation of liquid droplets in a supersaturated steam). Indeed, a small change in the surface tension is sufficient to change the nucleation rate by several orders of magnitude. ILM researchers have actually performed cavitation experiments in ethanol and heptane. Combining their measurements with cavitation data in water and condensation data in vapors of ethanol, heptane and water, they were able to show that the symmetric correction proposed by Tolman was not sufficient at the scales relevant to nucleation. This study suggests replacing the Tolman approximation, commonly used in nucleation, with a more complex correction (involving also the square of the radius of curvature) as suggested by recent simulations.