More highlights
|
Viscosity of supercooled waterSo far accurate viscosity data were lacking in supercooled water. Using Brownian spheres suspended in water, we have measured it down to −34°C. Whereas contrary to most fluids, viscosity decouples from molecular translation upon cooling, it remains coupled to rotation. This anormal behaviour could be related to a liquid-liquid phase transition, predicted by simulations. Viscosity of deeply supercooled water and its coupling to molecular diffusion, Dehaoui et al., PNAS (2015) |
Left : The setup. Brownian spheres suspended in supercooled water are placed in a thermal stage. Thanks to differential dynamic microscopy, the viscosity of water is deduced. Right : Example of obtained image. |
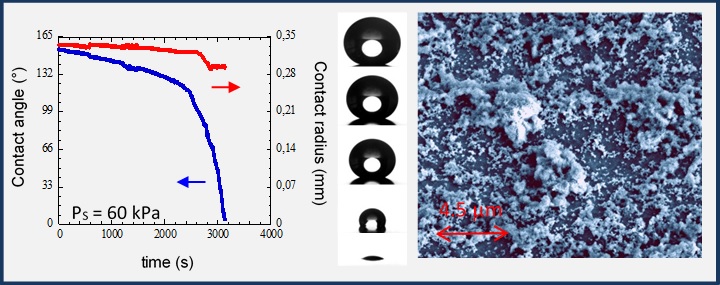
Effect of low pressure on the contact line dynamicsWe experimentally investigate the influence of the surrounding pressure Ps on evaporation dynamics of drops on (super) hydrophobic surfaces. Main results:(i) the friction at the liquid-solid interface decreases linearly with Ps on hydrophobic surfaces;(ii) under lower pressures a “fakir” to Wenzel transition occurs quasi-instantaneously. The pressure variation appears as a powerful tool to modify in a controlled way the liquid-solid interface. Sessile drop evaporation on (super)hydrophobic surfaces, Kiper et al., Colloids and Surfaces A (2015) |
SEM image of a processed sample, snapshots of an evaporating drop and temporal evolution of contact angle and contact radius. |
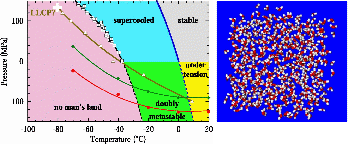
Anomalies of water at negative pressureSupercooled water exhibits pronounced anomalies. Their origin remains elusive because measurements are limited by crystallization of the system. By applying negative pressure and using Brillouin spectroscopy, we have observed a minimum in the sound velocity before crystallization occurs. Together with molecular dynamics simulations, these results put more constraints on theoretical scenarios proposed to explain water anomalies. Anomalies of bulk supercooled water at negative pressure, Pallares et al. PNAS (2014). |
Molecular dynamics simulations done in C. Valeriani’s group (Madrid) confirm the sound velocity measurements and allow to propose an explanation involving a putative phase transition in water between two different liquids. |
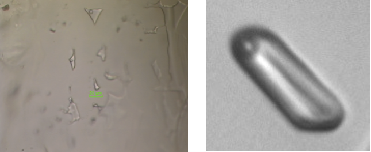
Water at negative pressureLiquide water under tension breaks into gaz: this is cavitation. To understand this phenomena, we have relied on a method where inclusions of liquid within a quartz crystal are cooled down at constant volume.The observed cavitation statistics are in good agreement with the classical mechanism of homogeneous cavitation and rule out other mechanisms. The results also enable us to locate the temperature of maximum density at negative pressure. This work is an advance on the phase diagram of water, which is still under debate. A coherent picture for water at extreme negative pressure, Mouna El Mekki Azouzi et al. Nature Physics (2012). |
Experiments performed up to now on these systems would measure the threshold for bubble appearance on many inclusions at the same time, whence a large dispersion in the result. To get very accurate measurements, we measured the threshold on a single, well-chosen inclusion (right), but repeated the process many times. |